Designed for the future: The Milliflex Oasis system for bioburden and water testing
Quality control in the pharmaceutical industry is a key component in the manufacturing of life saving drugs.
Monitoring the levels of microbial bioburden of raw materials, in-process samples, and final product requires products with precision. Accidental contamination (false positives) due to manipulation in the lab can lead to manufacturing slowdowns, financial losses, and potential drug delivery delays to patients in need.
As a result, the pharmaceutical industry is looking for options with fewer manipulations when performing bioburden testing. Merck is looking to the future when robotics will become a more prevalent option for companies to increase productivity, gain more control over testing steps, and increase traceability for each operational step performed. This is key to producing reliable results or identifying the root cause of out-of-specification results.
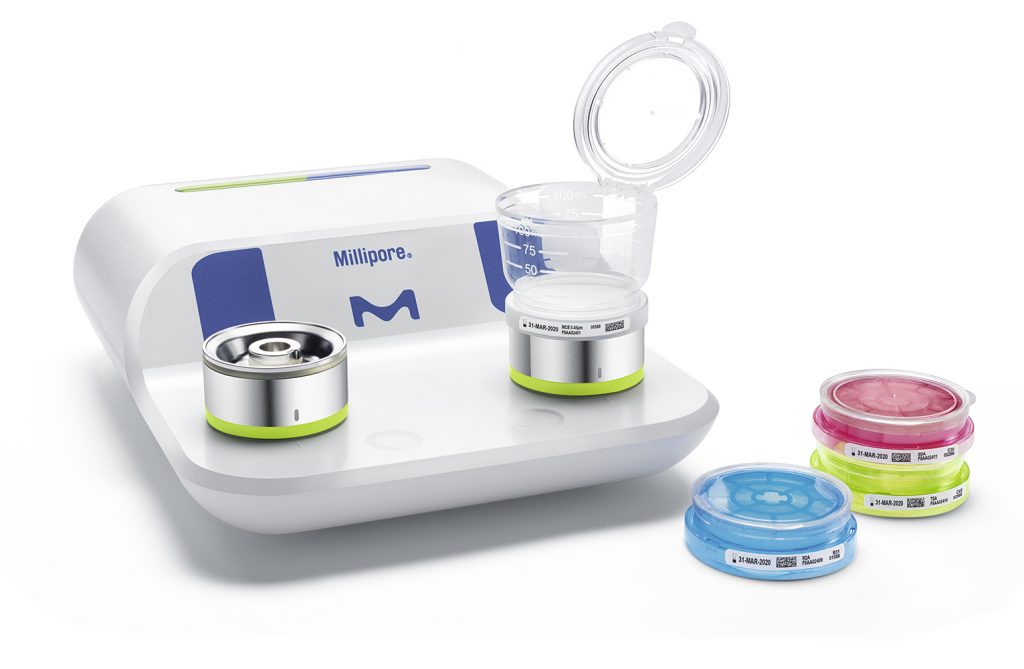
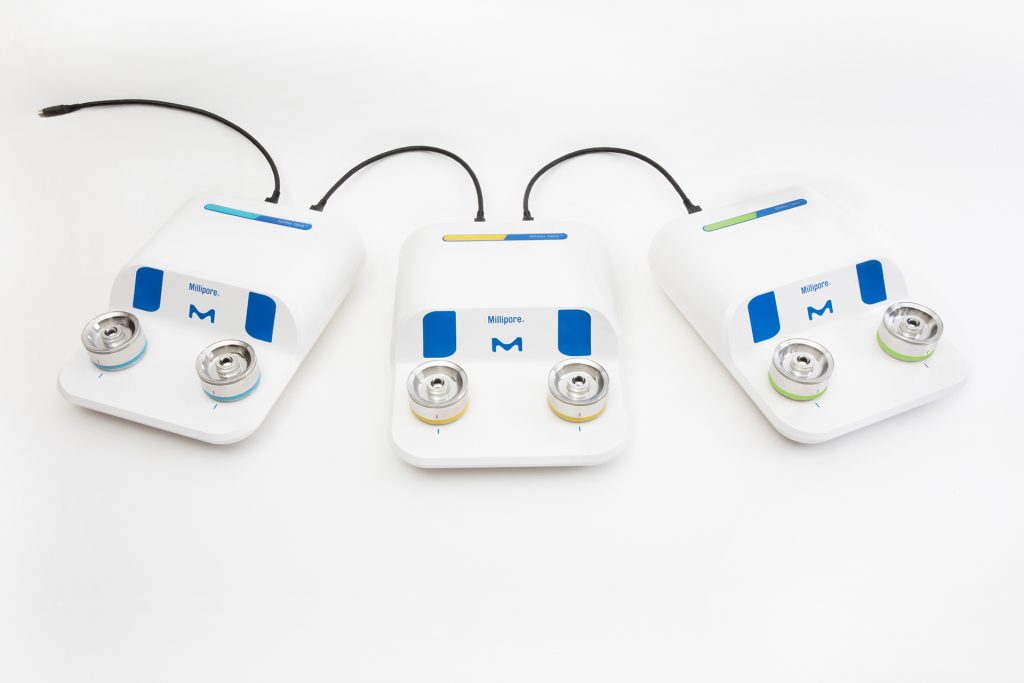
The Milliflex Oasis® system is a complete solution that includes the services for a secured workflow with the highest possible throughput. It includes:
- All-in-one filtration equipment
- Ready-to-use sterilized filtration units
- Pharmacopeia compliant culture media
Not only does the Milliflex Oasis® enhance laboratory efficiency but also provides compliance with international pharmacopeia standards while ensuring reliable results. Data integrity is critical for quality control laboratories. From the primary packaging to the individual product, the Milliflex Trace® feature ensures complete traceability by including detachable labels, a 2D barcode that can be scanned for direct access to documentation, and unique identification of each funnel and each media plate.
The most challenging testing step of the filtration method for quality control is transferring the membrane to the culture media. The Milliflex Oasis® system, unlike standard systems on the market, uniquely allows touch-free membrane transfer to the media plate. This protects the membrane from secondary contamination and ensures perfect contact between the membrane and the agar.
The new Milliflex Oasis® system has been designed for the current needs of industry and is preparing for the automation of the future.
Contact details:
Merck KGaA
Frankfurter Strasse 250
64293 Darmstadt, Germany
To place an order or receive technical assistance:
Order/Customer Service: SigmaAldrich.com/order
Technical Service: SigmaAldrich.com/techservice
Safety-related Information: SigmaAldrich.com/safetycenter
![]()